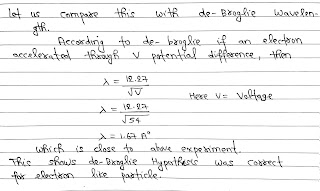
The de-Broglie suggested that every moving particle has a wave associated with it.
Davission and Germer experimentally proved that fast moving electron(particle) show diffraction, which is a property of of wave.
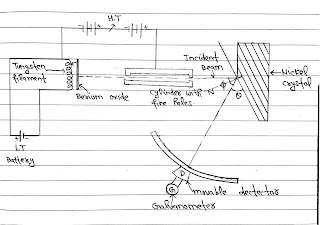
Experimental Setup and Construction:
It consists of an electron gun which compares of a tungsten filament F, coated with barium oxide and heated by a low voltage power supply. Electrons emitted by the filament are associated to a desired velocity by applying suitable potential from a high voltage power supply.
They are made to pass through a cylinder with free holes along its Axis, producing a fine collimated beam. The beam is made to fall on the surface of a Nickel crystal. The electrons are scattered in all directions by the atom of the Crystal, which cut along cubicle Axis at a particular angle.
The scattered beam of electrons is received by the detector which can be rotated at any angle and it connected with a galvanometer.
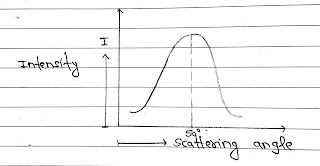
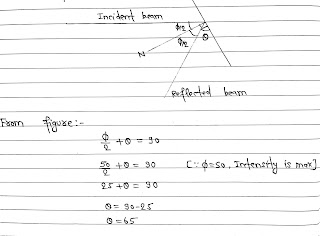
Working: