According to Planck’s Quantum theory, light radiations consists of tiny packets of energy called quanta.
One quantity of light radiation is called a photon which travels with the speed of light.
The energy of a Photon is E= hv
Where h is Planck’s Constant and v is frequency of light radiation.
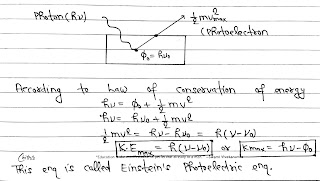
When a photon of energy fall on a metal surface, the energy of photon is absorbed by the electron and is used in following two ways.
(I) A part of energy is used to overcome the surface areas and electron comes out of the metal surface this part of energy is called work function (Ø₀) and Ø₀= hv₀
(II) The remaining part of the energy is used in giving a velocity to the photoelectron. This is equal to the maximum kinetic energy of photoelectron.
* If v<v₀, no emission will take place.
* If v= v₀, emission will take place but photo electron have no any kinetic energy.
* If v>v₀, emission will take place.