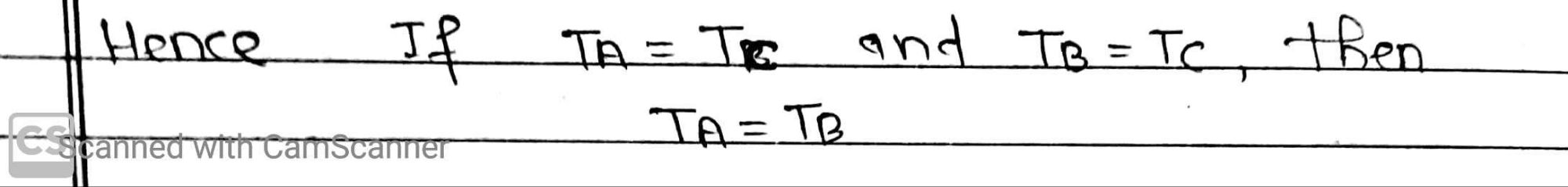This law was formulated by RH Fowler in 1931. The Zeroth law of thermodynamics state that if two system A and B are separated in thermal equilibrium with a third system C, then A and B in thermal equilibrium with each other.
If two system A and B separated by an adiabatic wall. They are separated from system C by a diathermic wall. The system A and C, B and C will reach thermal equilibrium separately. If adiabatic wall between A and B is removed, there will be no exchange of heat showing that A and B are already in thermal equilibrium.

Concept Of Temperature
Zero law of thermodynamic implies that temperature is a physical quantities which has the same value for all system which are in thermal equilibrium with each other.