Energy Bands In Solids (Conductor, Insulator and Semiconductor)
According to Bohr atomic model and concept of electronic configuration in an isolated atom, there are well defined energy levels of electrons in an isolated atom.

But in real, isolated atom is not possible. Many atom comes together and form a crystal. In case of crystal, these atom come together nearly 2 to 3 A⁰ and they are no more isolated.
In a crystal due to inter atomic interaction valence electron of one atom are shared by more than one atom in the crystal.
Now, splitting of energy level takes place. The collection of these closely spaced energy levels is called energy bands.
Energy bands are formed due to the continuous energy variation in different energy levels.
These different energy levels in different electrons are formed because inside the crystal, each electron has a unique position and no two electrons is exactly at the same pattern of surrounding charges.
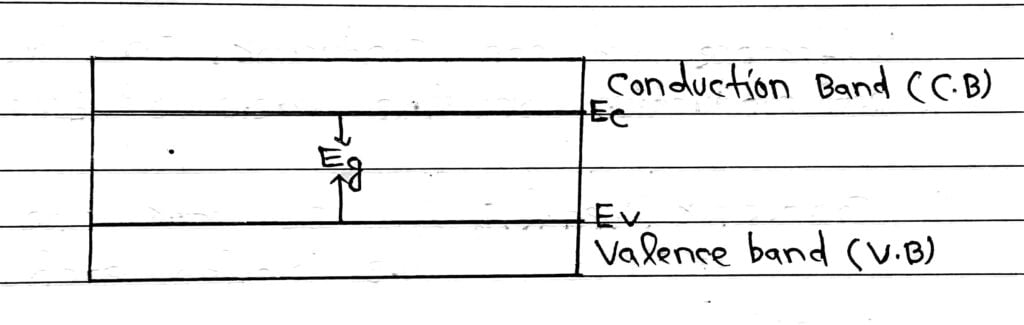
Valence Band
The highest energy band filled with valence electron is called valence band. This band may be partially or completely filled with electrons. This band is never empty.
Conduction Band
The lowest unfilled allowed energy band next to valenced band or above the valence band is called conduction band.
Energy Band Gap or Energy Gap
Sometime known as Forbidden Energy Gap.
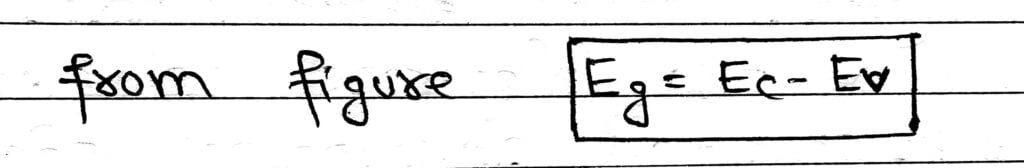
The gap between top of valence band and bottom of the conduction band in which no allowed energy levels for electrons can exist is called energy band gap.
Also Read: CBSE Class 12 Physics Chapter-14 (Semiconductor and Electronic Devices) All Topics
