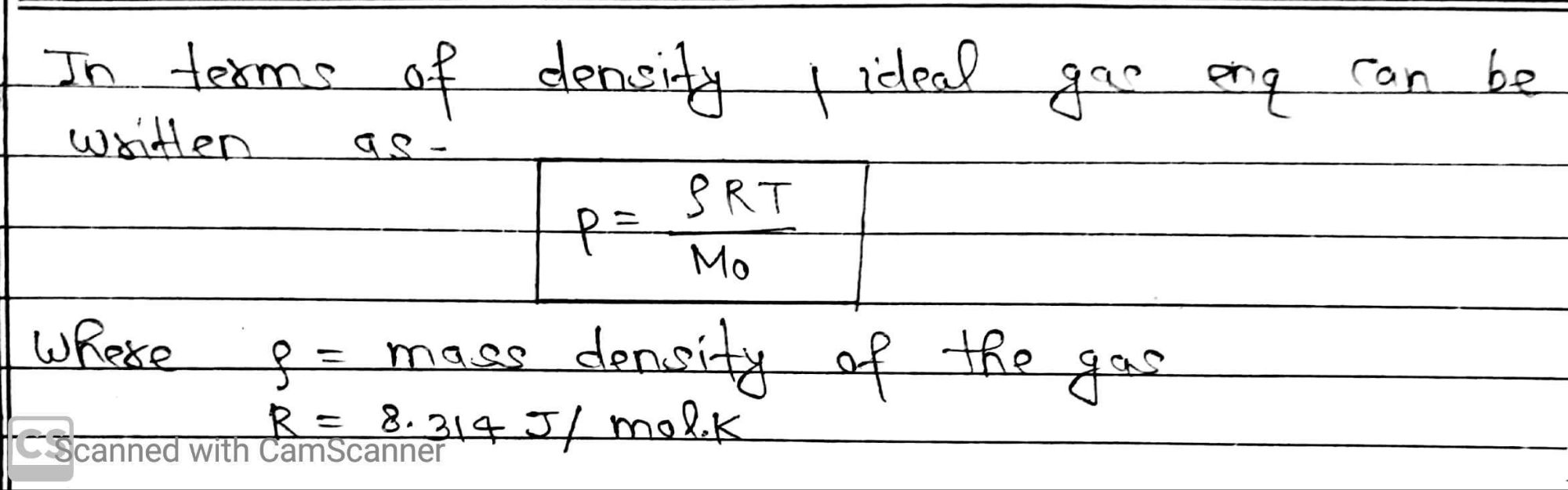
The molecules of gas are far from each other and their mutual interactions are negligible except when two molecules collide. So, gases at low pressure and high temperature much above that at which they (liquify or solidify) follow a relation
pV = KT -(i)



Behaviour Of Real Gas and Ideal Gas
An ideal gas is a theoretical model of a gas and no real gas is truly ideal. However, a real gas behaves as an ideal gas most closely at low pressure and high temperature. This is because at low pressure and high temperature, the molecules of gas are apart due to which molecular force of attraction is negligible.
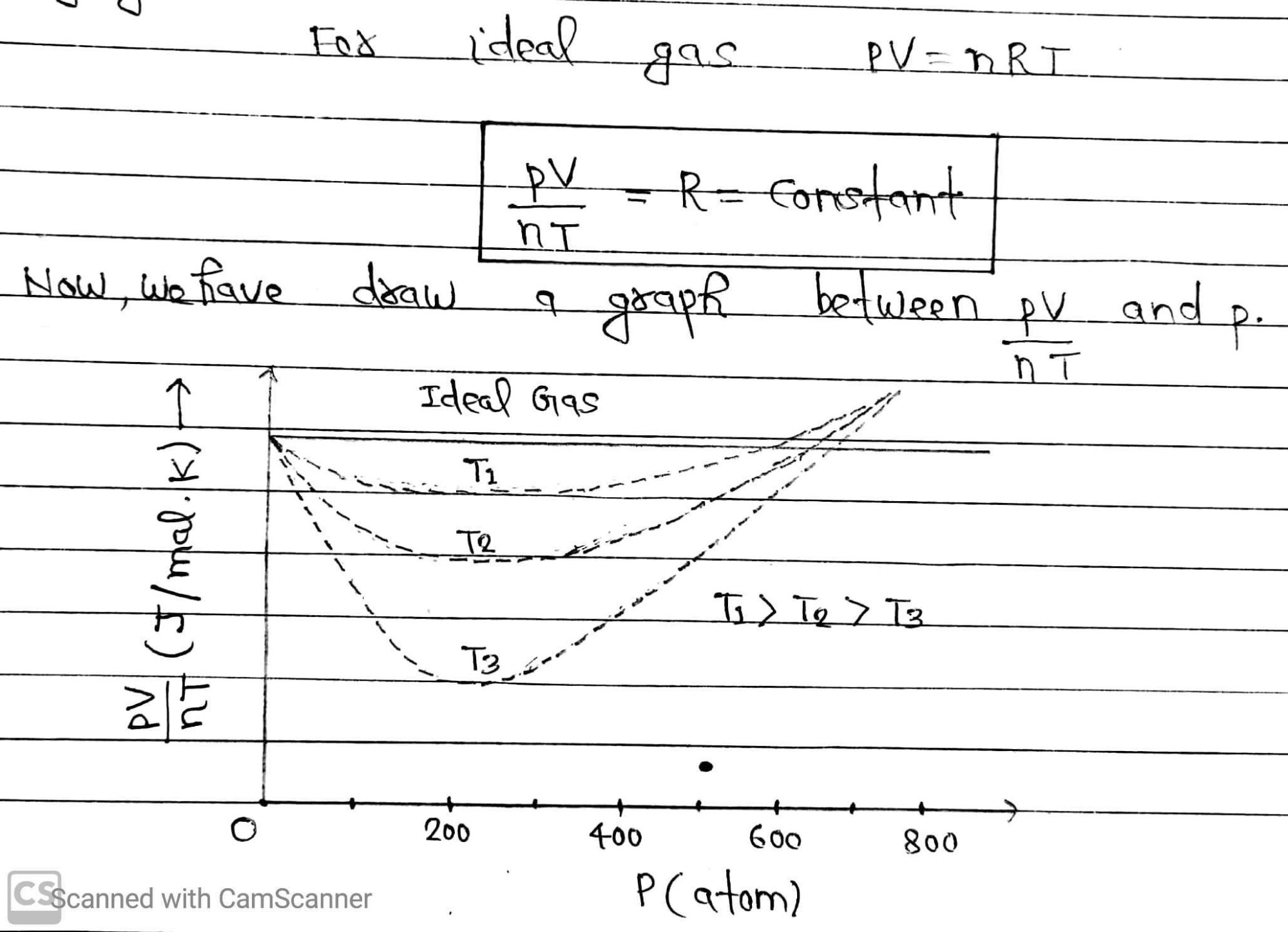
If we plot same graph for real gases at three different temperature T₁,T₂ and T₃ (T₁>T₂>T₃), then we find the curve as dotted lines.
Solid line showing ideal gas approach and dotted line showing real gas approach. From the observation of this graph, we will find that all curves for real gas approach ideal gas behaviour at low pressure and high temperature.
