Distance of Closest Approach:
As the Distance particle approaches the nucleus, the electrostatic force of repulsion due to nucleus increases and the kinetic energy of α particle goes on converting into the electrostatic potential energy.
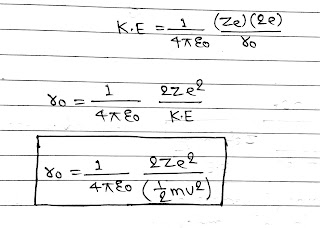
At a certain distance r₀ from the nucleus, whole of the kinetic energy of α particle convert into electrostatic potential energy and α particle cannot go further close to nucleus, this distance(r₀) is called distance of closest approach.
Note:
Since, V= w/q
Vq= w
Vq= Potential Energy and We know that
V= q/4πε₀r
Here z= atomic number which is equal to number of proton in nucleus and e= charge of proton, So total charge= Ze
In α particle there are total 2, +Ve charge. So if charge on 1, α particle be e, then
Total Charge = 2e.
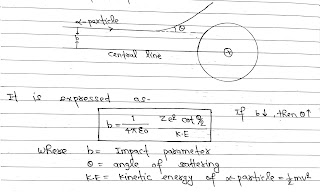
Impact Parameter:
Perpendicular distance of the velocity vector of α particle from the central line of the nucleus of the atom is called Impact Parameter.
Electron Orbits:
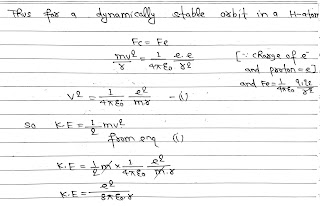
The Rutherford nuclear model of the atom pictures the atom as an electrically neutral sphere consisting of a very small, massive and positively charged nucleus at the centre surrounded by the revolving electron in their respective dynamically stable orbits.
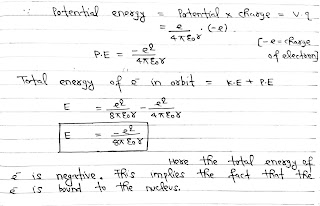
The electrostatic force(Fb) between the revolving electrons and the nucleus provides the requisite centripetal force(Fc) to keep them in their orbits. (like Moon and Earth gravitational and centripital).