Heat is the form of energy which flows from hotter body to colder body by virtue of temperature difference. The process of transfer of heat is a non-mechanical process i.e there is no mechanical work involve in the process of heat transfer. The amount of heat is measured in joule (SI unit) and other widely used unit for the heat is calorie (in CGS).
1 joule = 4.2 Calorie (Cal)
1 calorie is equal to the amount of heat energy required to rise the temperature of 1 gram of water through 1 ⁰C (From 14.5 ⁰C to 15.5 ⁰C).
There are two theory of heat.
(I) Caloric Theory Of Heat
According to this theory, heat is an invisible, weightless and odourless fluid called caloric. When some caloric is added to a body, its temperature rises, and when some caloric is removed, its temperature falls.
(II) Dynamic Theory Of Heat
According to this theory, all substances (solid, liquid and gas) are made of molecules. These molecules are in a state of continuous random motion. Depending on the nature and temperature of the substance the molecules may process three types of motion.
(a) Translatory Motion
This is the motion in a straight line which is common in gases.
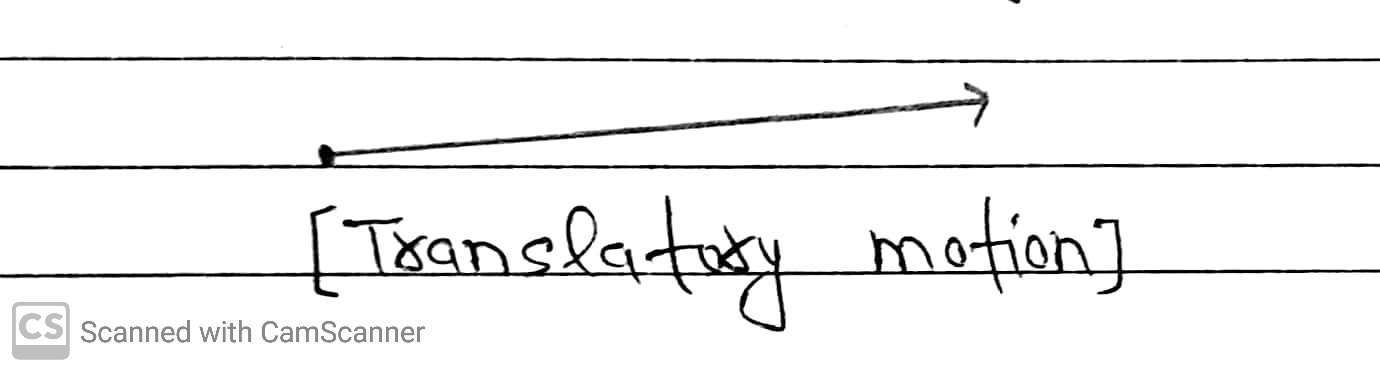
(b) Vibrational Motion
This is to and fro motion of the molecules about their main positions. This is common in liquid and gas.

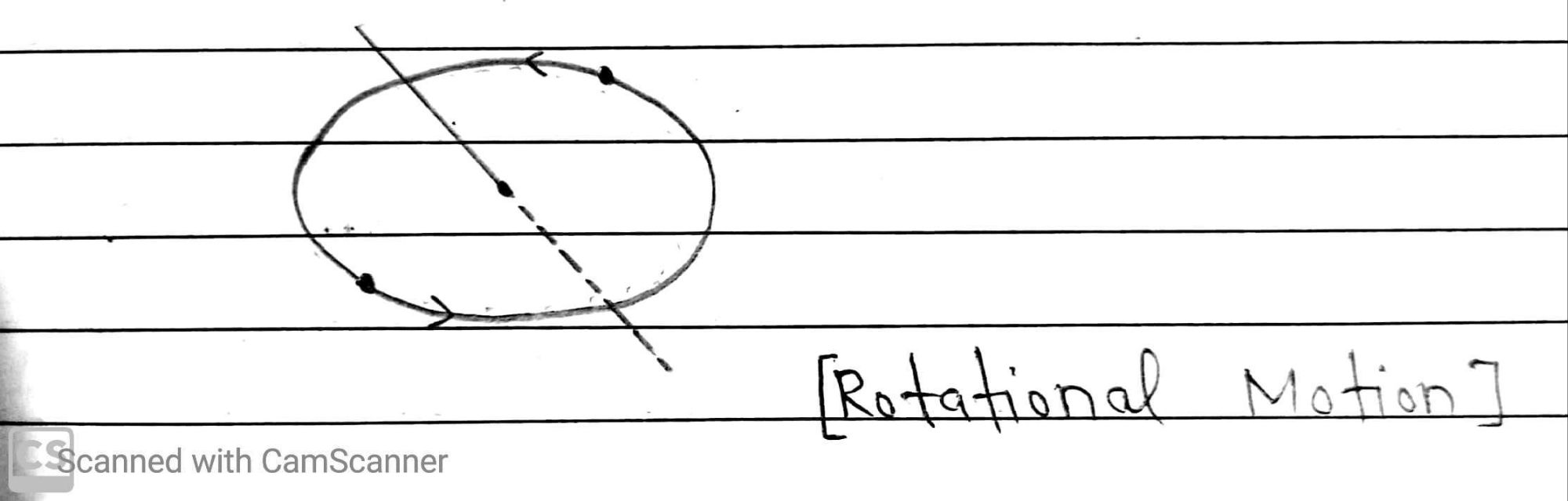
(c) Rotational Motion
This is the motion of molecules about their axis. This type of motion occurs usually at high temperature.