There are three characteristics of gas which describe its behaviour. These are pressure(P), volume(V) and temperature(T).
The relation among pressure, volume and temperature of a gas is given by these two laws.
(1) Boyle’s Law
It is given by English chemist Robert Boyle (1627-1691). According to this law at constant temperature, pressure is inversely proportional to volume.

(2) Charles Law
This law is given by French Scientist Jacques Charles (1747-1823).
According to this law at constant pressure, volume is directly proportional to temperature.
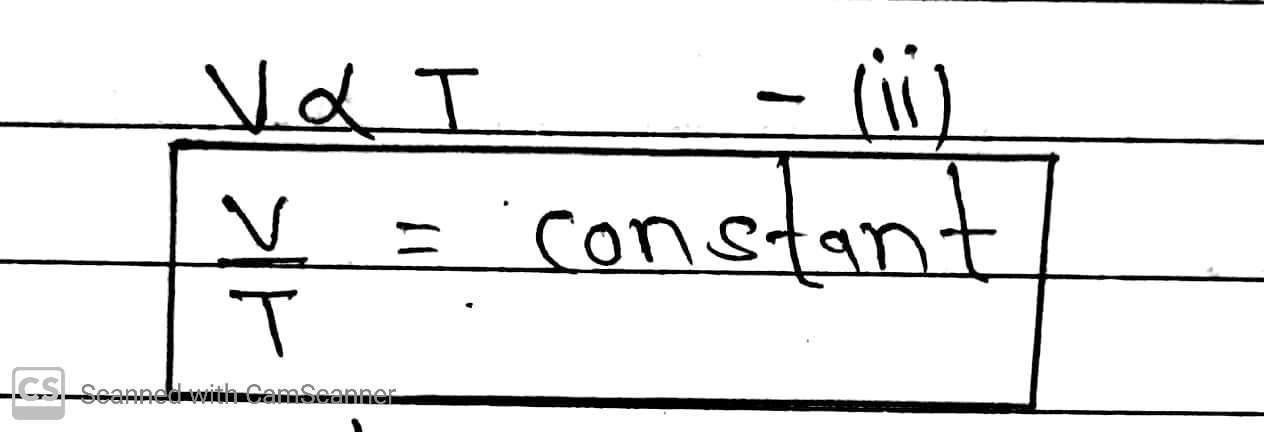
Ideal Gas Equation
On combining above two laws, we get

