A steel needle may be made to float on water through the Steel is more dense than water. This is because the water surface acts as a stretched elastic membrane and supports the needle. This property of a liquid is called surface tension.
Surface tension is the property by virtue of which the free surface of a liquid at rest behaves like an elastic stretched membrane tending to contract so as to occupy minimum surface area.
We imagine a line AB on the free surface of a liquid. The small elements of the surface on this line are in equilibrium because they are acted upon by equal and opposite forces, acting perpendicular to the line from either side. The force acting on this line is proportional to the length of this line.
If l is the length of the imaginary line and F the total force on either side of the line, then
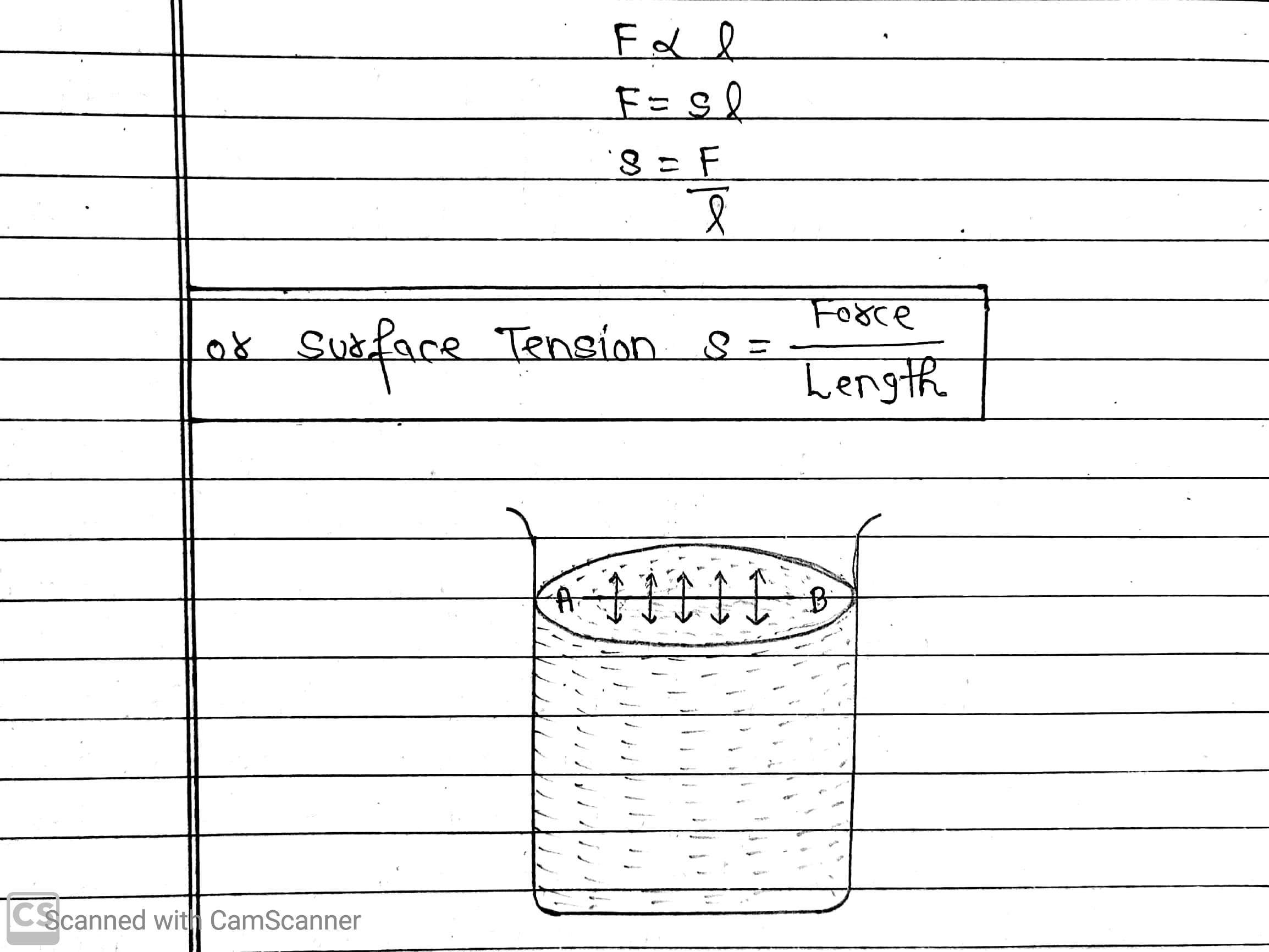
Surface tension is measured as the force acting per unit length of an imaginary line drawn on the liquid surface the direction of force being perpendicular to this line and tangential to the liquid surface.
Unit and Dimension Of Surface Tension
SI unit of surface tension is N/m. CGS unit of surface tension is dyne/cm and dimensional formula is [MLT].
Factor Affecting Surface Tension
(I) Temperature
The surface tension of liquid decreases with rise in temperature.
(II) Addition Of Impurities
The surface tension of liquids changes appreciably with addition of impurities.
Detergent and Surface Tension
Surface tension has a wide use in daily lif The detergents used for cleaning the dirty clothes in our home is a very good example of surface tension.
Actually, water can not wet oil stain on our clothes, that is why water alone cannot remove dirt from our clothes.
The molecule of detergent can get attached with water and dirt molecules and they take away the dirt with them when we wash the clothes with detergent.
Application Of Surface Tension
(I) Rain drops and drops of Mercury placed on glass plate are spherical.
(II) Hair of shaving brush/painting brush, when dipped in water spread out, but as soon as, it is taken out, its hair stick together.
(III) A greased needle placed gently on the free surface of water in a beaker does not sink.
(IV) Oil drop spreads on cold water, but does not change shape on hot water.
