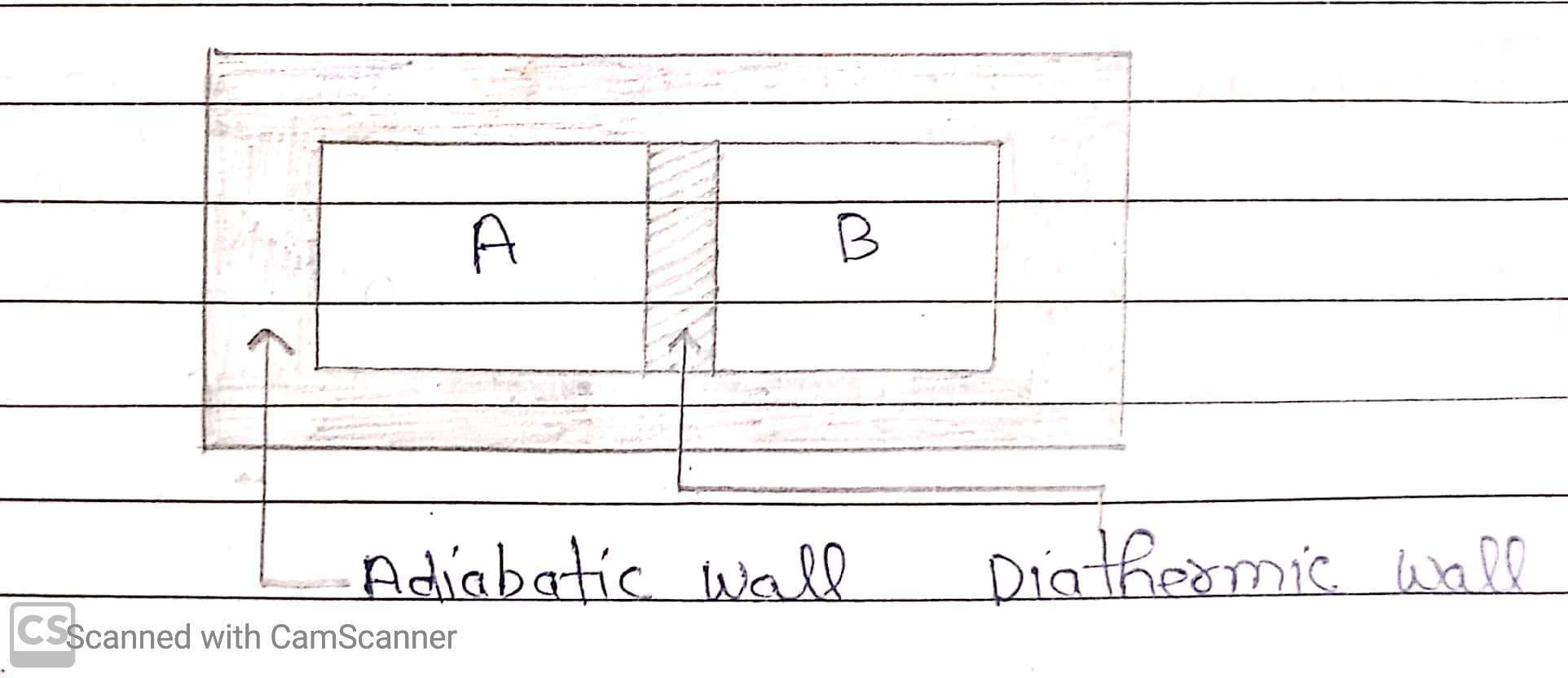
Two systems are said to be in thermal equilibrium with each other, if they have the same temperature.
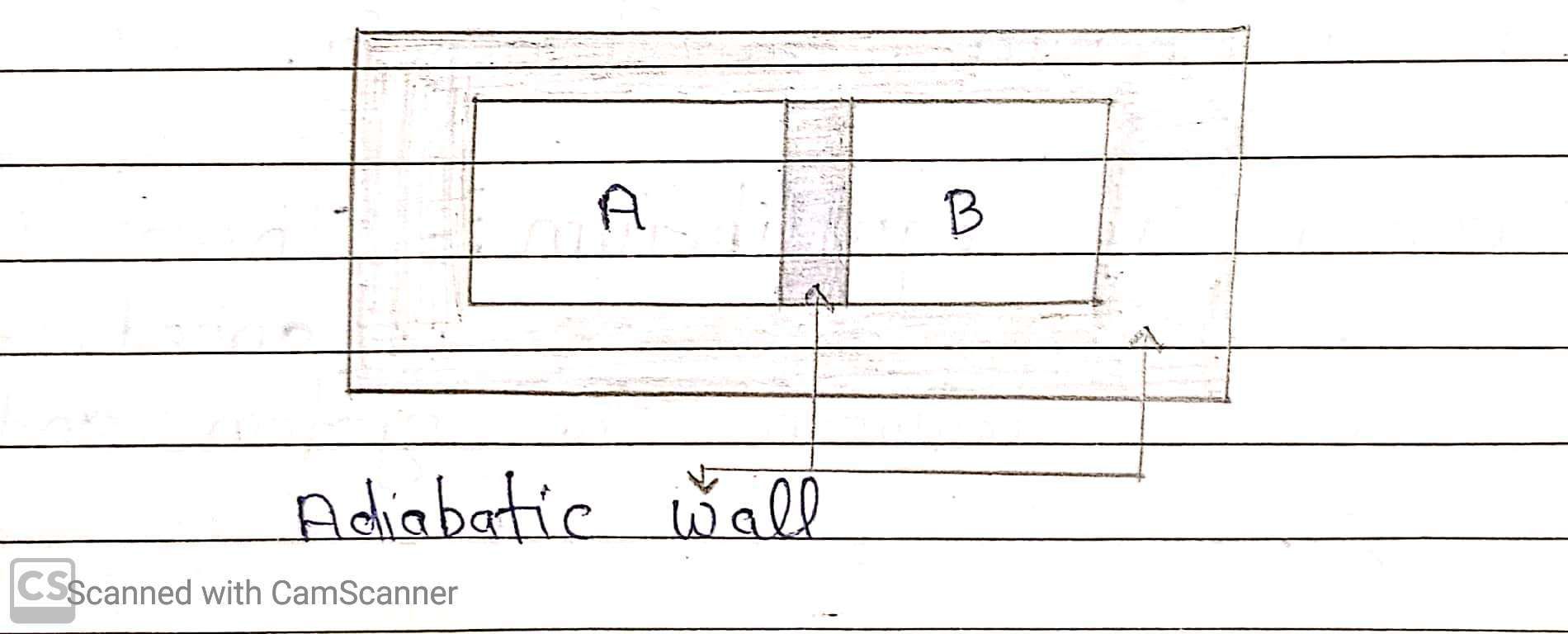
Let two gases A and B contained in two different vessels. Let the pressure and volume of the gases be (PA and PB) and (VA and VB) respectively. If the two vessels are separated by an adiabatic wall, then any possible values (PA, VA) will be in equilibrium with any possible pair of values (PB, VB).
If A and B are now separated by a diathermic wall, than the pressure and volume variables of the two gases changes to (PA’, VA’) and (PB’, VB’) such that the new state of A and B are in equilibrium with each other. There is no more flow of heat. The two system attain equal temperature and we say that they are in equilibrium with each other.