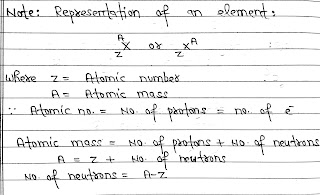
Atomic Number:
Atomic number of an element is the number of proton present inside the nucleus of an atom of the element. It is also equal to the number of electron revolving around the various orbits.
Atomic Number Z = Number of protons = Number of electrons(in neutral atom).
Mass Number:
Mass of an element is the total number of protons and neutrons inside the atomic nucleus of the element.
Mass Number or Atomic Mass = Number of Protons+Number of Neutrons
Classification of Nuclei:
1.Isotopes: Atoms of an element whose nuclei have same atomic number but different atomic mass.
2.Isobars: Atoms of different elements whose nuclei have same number of nucleon but different number of protons and neutrons.
3.Isotones: Atoms of different elements whose nuclei have same number of neutrons.