CBSE Class 12 Chemistry Term-2 2022: 3 Marks Important Questions With With Answers
In this post I have provided CBSE Class 12 Chemistry Term – 2 most Important 3 Marks Important Questions with Solutions for Board Exam 2022. As we know CBSE will conduct Term 2 Chemistry Board Exam for class 12 on 7 May 2022. Here all of you can check for additional 3 marks important questions which are given below to solve before going for your CBSE Class 12th Term 2 board exam 2022.
Read More: CBSE Class 12 Chemistry Term 2 Sample Paper

Given below are the CBSE Class 12 Chemistry term 2 important questions with solutions/answer.
Read More: CBSE Class 12 Subject-Wise Sample Paper From Official Website
Que 1. A solution of Ni(NO3)2 is electrolysed between platinum electrodes using a current of 5 amperes for 20 minutes. What mass of Ni is deposited at the cathode?
Answer : Given,
Current = 5A
Time = 20 × 60 = 1200 s
Charge = current × time
= 5 × 1200
= 6000 C
According to the reaction,
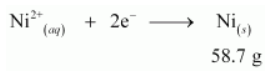
Nickel deposited by 2 × 96487 C = 58.71 g
Therefore, nickel deposited by 6000 C = (58.71 X 6000) / (2 X 96487) g
= 1.825 g
Hence, 1.825 g of nickel will be deposited at the cathode.
Que 2. State and explain Faraday’s first law of electrolysis.
Answer : Faraday’s first law of electrolysis:
The amount of the substance that undergoes oxidation or reduction at each electrode during electrolysis is directly proportional to the amount of electricity that passes through the cell.
Thus mass of the substance produced

Here I(A) is current in ampere and t(s) is time in second.
Que 3. Differentiate between metallic conductor and electrolytic conductor. Discuss the effect of temperature on their conductivity.
Answer :
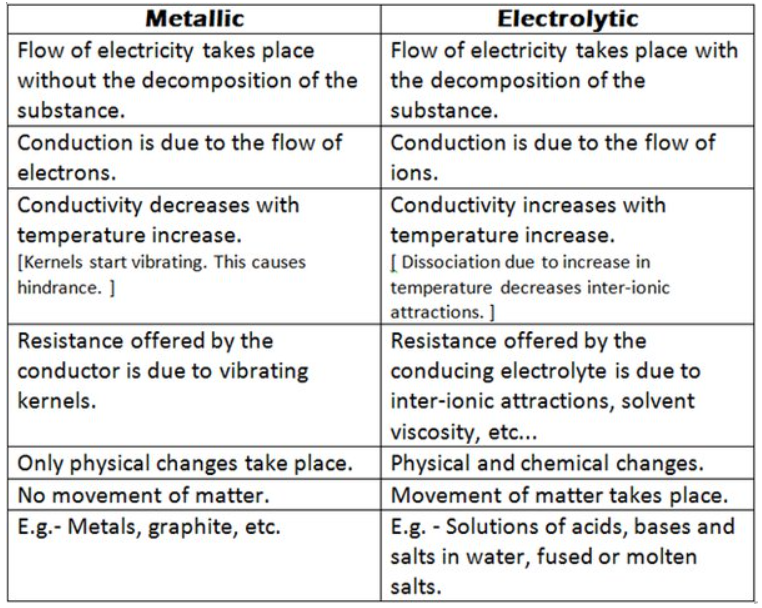
When we increase the temperature, the kinetic energy of the ions increases and they move faster i.e. they conduct their bearing charge faster and thus result in increased conductivity.
So, with increase in temperature, the conductivity of electrolytic conductors increases.
Read More: Best CBSE Class 12 Chemistry Study Materials
Que 4. Write the order of a chemical reaction, which proceeds at a uniform rate.
Answer : Zero order reaction proceeds with a uniform rate throughout.
rate = k[A]0 = k = constant.
The rate of the reaction is independent of the reactant concentration.
Que 5. Differentiate between the order and molecularity of a reaction.
Answer : The molecularity of a reaction represents the number of atoms, molecules, or ions that will interfere with each other for the chemical reaction to occur within a limited time frame. The main differences between order and order of reaction are given below.
| Molecularity | Order |
| The number of ions or molecules that take part in the rate-determining step is known as molecularity. | The sum of powers to which the reactant concentrations are raised in the rate law equation is known as the order of the reaction. |
| The molecularity of a reaction is a whole number other than zero. Its values are limited from 1 to 3. | It can either be a whole number or a fraction. Therefore, it can be 0, 1, 2, 3 or even a fraction. |
| The molecularity of the reaction is determined by looking at the reaction mechanism | The order of the reaction is determined by the experimental methods |
| The molecularity of the reaction is obtained by the rate-determining step | The order of the reaction is obtained by the sum of the powers to which the reactant concentrations are raised in the rate law equation |
Que 6. What are detergents? Give their scheme of classification. Why are detergents preferred over soaps?
Answer : Detergents are sodium salts of long chain sulphonates and sulphates.
Classification: They are two types:
(i) Sodium salts of long chain benzene sulphonic acid: These are obtained from derivatives of benzene sulphonic acid. The common example is sodium p-dodecyl benzene sulphonate.
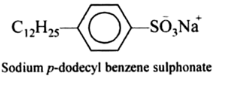
(ii) Sodium salts of long chain alkyl hydrogen sulphate: These are sodium salts of sulphuric acid esters of long-chain alcohols containing usually 10-15 carbon atoms. These alcohols are obtained by the hydrogenolysis of oils and fats. For examples, sodium dodecyl sulphate or sodium lauryl sulphate and sodium cetyl sulphate.

Detergents are preferred our soaps for the following reasons:
(i) Synthetic detergents can be used even in the case of hard water whereas soaps fail to do so.
(ii) Synthetic detergents can be used in the acidic medium while soaps fail to do so because of their hydrolysis to free acids.
(iii) Synthetic detergents are more soluble in water and hence form more latter than soaps.
(iv) Synthetic detergents have a stronger cleansing action than soaps.
Que 7. Define adsorption and write two important differences between physical adsorption and chemisorption.
Answer : Adsorption is a process that involves the accumulation of a substance in molecular species in higher concentrations on the surface. If we look atHydrogen, Nitrogen and Oxygen, these gases adsorb on activated charcoal. Meanwhile, we have to note that adsorption is different from absorption. The two processes involve totally different mechanisms.
For the adsorption process, two components are required,
- Adsorbate: Substance that is deposited on the surface of another substance. For example,H2, N2 and O2 gases.
- Adsorbent: Surface of a substance on which adsorbate adsorbs. For example, Charcoal, Silica gel, Alumina.
The difference between physical and chemical adsorption are tabulated below.
| Physical adsorption | Chemical Adsorption |
| This type of adsorption is also known as physisorption. | This type of adsorption is also known as chemisorption. |
| It is due to weak Van der Waals forces between adsorbate and adsorbent. | It is due to strong chemical forces of bonding type between adsorbate and adsorbent. |
| This adsorption is a multi-layered process. | This type of adsorption is almost a single-layered phenomenon. |
| Surface area, temperature, pressure, nature of adsorbate effects physisorption | Surface area, temperature, nature of adsorbate effects chemisorption. |
| Examples: H2 and N2 gases adsorb on coconut charcoal | Examples: Charcoal, Silica gel, Alumina. |
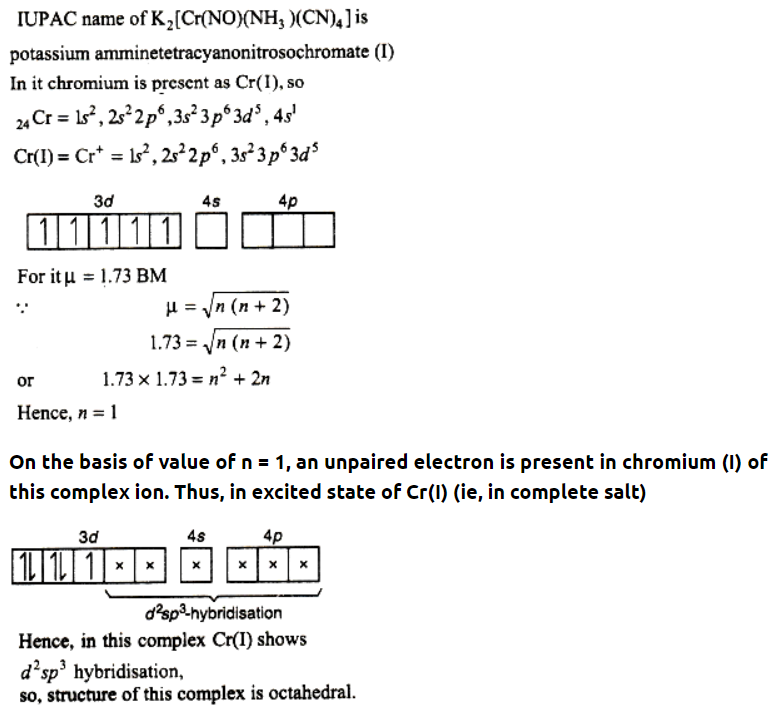
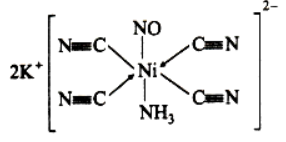
Que 8. Write the IUPAC nomenclature of the given complex along with its hybridisation and structure
K2[Cr(NO)(NH3)(CN)4], μ = 1.73.
Answer :
Que 9. Draw a figure to show the splitting of d orbitals in an octahedral crystal field.
Answer :
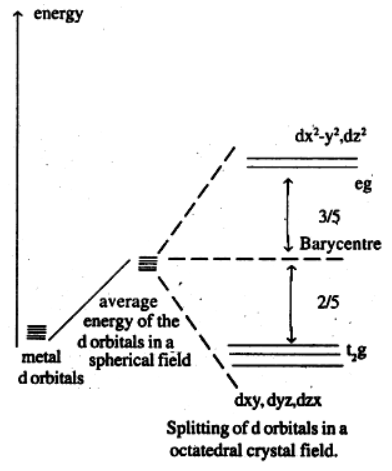
The splitting of d orbitals in an octahedral field takes place in such a way that dx2-y2, dz2 experience a rise in energy and form the e.g. level, dry, dyz, dzx experience a fall in energy and form t2g level.
Que 10. Account for the following:
- Propanol has a higher boiling point than Butane.
- Ortho-nitrophenol is more acidic than ortho-methoxy phenol.
- Preparation of ethers by acid dehydration of secondary or tertiary alcohols is not a suitable method.
Answer : (i) It is because propanol can form intermolecular hydrogen bonds.
(ii) It is because –NO2 group is electron withdrawing and –OCH3 group is electron releasing. Therefore o-nitrophenoxide is more stable than o-methoxyphenoxide ion.
(iii) It is because secondary and tertiary alcohols on dehydration lead to the formation of alkene and not ethers due to stability of secondary and tertiary carbocation.
Que 11. Account for the following:
- The boiling points of alcohols decrease with an increase in the branching of the alkyl chain.
- Phenol does not give protonation reaction readily.
- Phenylmethyl ether reacts with HI to give Phenol and Methyl iodide and not Iodobenzene and methyl alcohol.
Answer : (i) The boiling point of alcohols decrease with increase in branching of alkyl chain: This is because of attraction decreases with decrease in surface area, hence boiling point decreases.
(ii) Phenol does not give protonation reaction readily: In phenols, the Ione pair of oxygen is being shared with benzene ring through resonance. Hence the electron density around oxygen is relatively less and therefore penols does not give protonation easily.
(iii) Phenyl methyl ether reacts with HI to give phenol and methyl iodide and not iodobenzene and methyl alcohol,
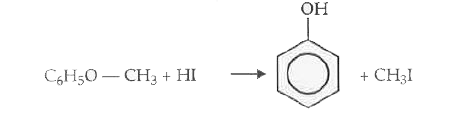
It is probably due to the fact that phenyl oxygen bond has a partial double bond character due to resonance. Therefore its cleavage is difficult as compared to alkyl oxygen bond.
