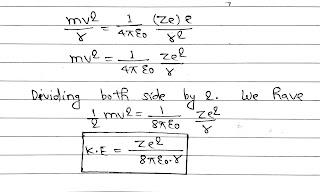
As we know that the centripetal force is provided by the electrostatic force of attraction.
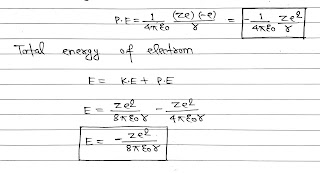
The potential energy of the electron in an orbit of radius r due to the electrostatic attraction by the nucleus is
The negative energy of electrons shows that the electron is bound to the nucleus and is not free to leave it.
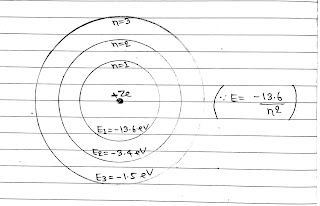
Energy Levels:
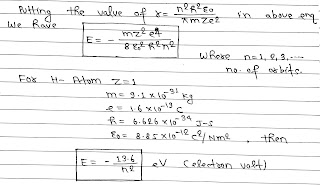
The energy of an electron is least, when its electron is revolving in an orbit closest to the nucleus. The lowest state of the atom is called the ground state. This state has lowest energy. The energy of this state is -13.6 eV.