The strong forces of attraction which hold together the nucleus in small nuclei of an atom inspite of electrostatic forces of repulsion.
From binding energy curve, We have seen that for average mass nuclei, The binding energy per nucleon is approximately 8 MeV, Which is much larger than the binding energy in atoms. Thus, for binding a nucleus together, Their must be a strong attractive force of a totally different kind.
This force must be strong enough to overcome the repulsion between protons and to bind both protons and neutrons into tiny nuclear volume.
Since this nuclear force is much larger than electrostatic force between protons (repulsive) that is why protons in nucleus are stable.
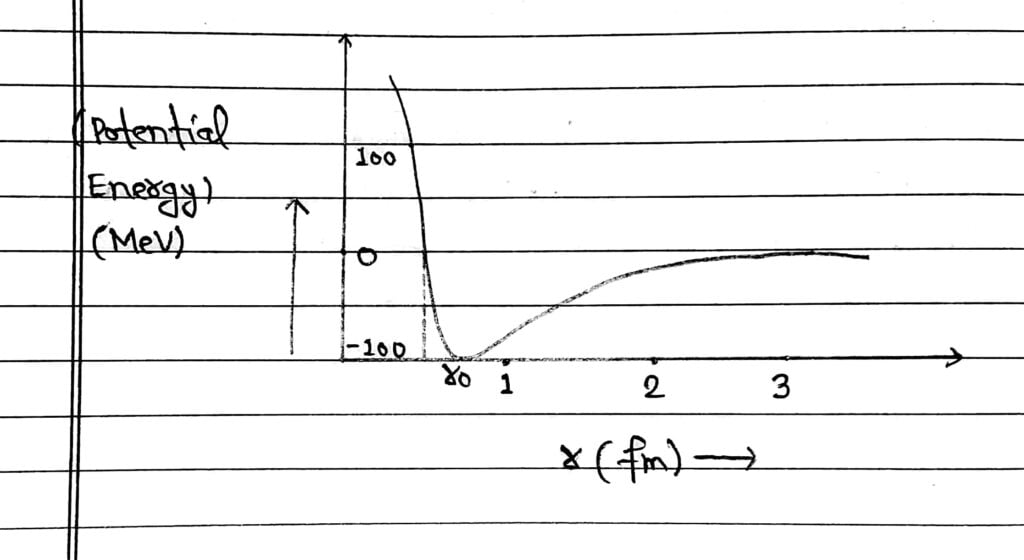
From the graph, it is concluded that potential energy is minimum at the distance r₀ ≈ 0.8 fm which means, the force is attractive for distances larger than 0.8 fm and repulsive for the distances less than 0.8 fm between nucleons.
Characteristics Of Nuclear Forces
1. Nuclear forces acts a pair of neutrons, a pair of protons and also between a neutron and proton. Therefore we can say that nuclear forces are charge independent.
2. They are strongest forces in nature.
3. They are very short range force.
4. They do not follow Inverse Square Law like electrostatic force.
5. The nuclear forces are independent on spin or angular momentum of nuclei.
