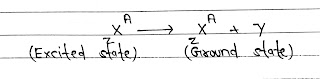Radioactive Displacement Laws:
This Law describes which chemical element and isotope are created during the particular type of radioactive decay.
α-Decay:
α-Decay is a process in which and unstable nucleus transforms itself into a new nucleus by emitting and α particle (A Helium nucleus ₂He⁴).
Since an α particle has two protons and two neutrons, so after an α-Decay, the present nucleus is transformed into a daughter nucleus with mass number smaller by 4 and atomic number smaller by 2.
Decay is possible only, when the total mass of decay products is less than the mass of the initial nucleus. This difference in mass appears as kinetic energy of the products.
β-Decay:
The process of spontaneous emission of an electron(e⁻) or a positron(e⁺) from a nucleus is called β-Decay.
The emission of electron (β⁻-decay) is accompanied by the emission of antineutrino(v⁻) and emission of positron(β⁺-decay) is accompanied by neutrino(v).
Neutrinos are neutral particles with mass approx zero.
β⁻-decay:
The mass number of the radioactive nucleus remains unchanged but its atomic number increases by 1. An electron and a new particle antineutrino are emitted from the nucleus.
β⁺-decay:
The mass number of the radioactive nucleus remains unchanged but its atomic number decreases by 1. A positron and a new particle neutrino are emitted from the nucleus.
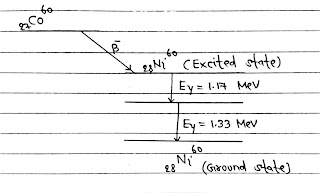
γ-Decay: