The atoms in a solid are held together by interatomic or intermolecular forces. These forces keep solid in a stable equilibrium position. When a solid is deformed, the atoms or molecules are displaced from their equilibrium positions. Thus, deformation causes change in interatomic or intermolecular distances.
On removing deforming forces, the inter atomic forces tend to drive the displaced atoms or molecules to their original equilibrium positions.
Spring Ball Model Of Solids
Atoms in a solid may be regarded as mass points or small balls connected in three dimensional shape through springs. Then, springs represent the interatomic forces of attraction between balls. This is called spring Ball model of a solid.
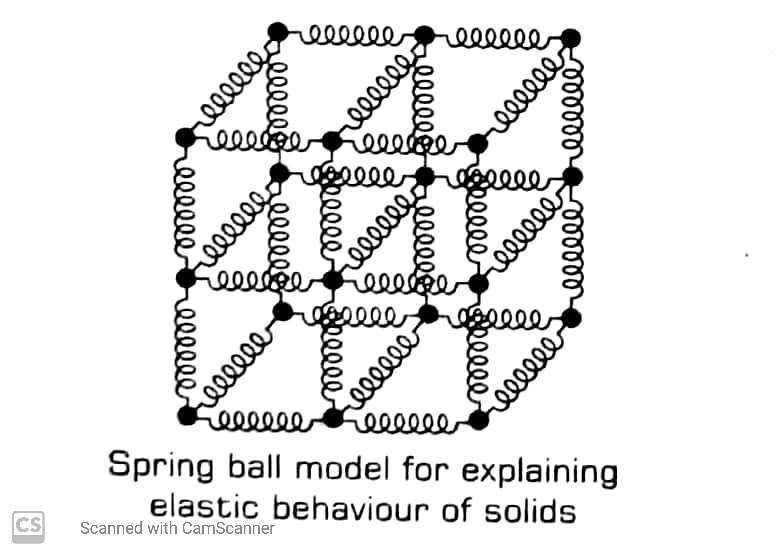
Normally, the balls occupy the positions of minimum potential energy or zero interatomic force. When any ball is displaced from its equilibrium or mean positions, the various springs connected to it exert a restoring force on this ball. This force tends to bring the ball to its equilibrium position. It shows the elastic behaviour of solid in terms of microscopic nature of the solid.
